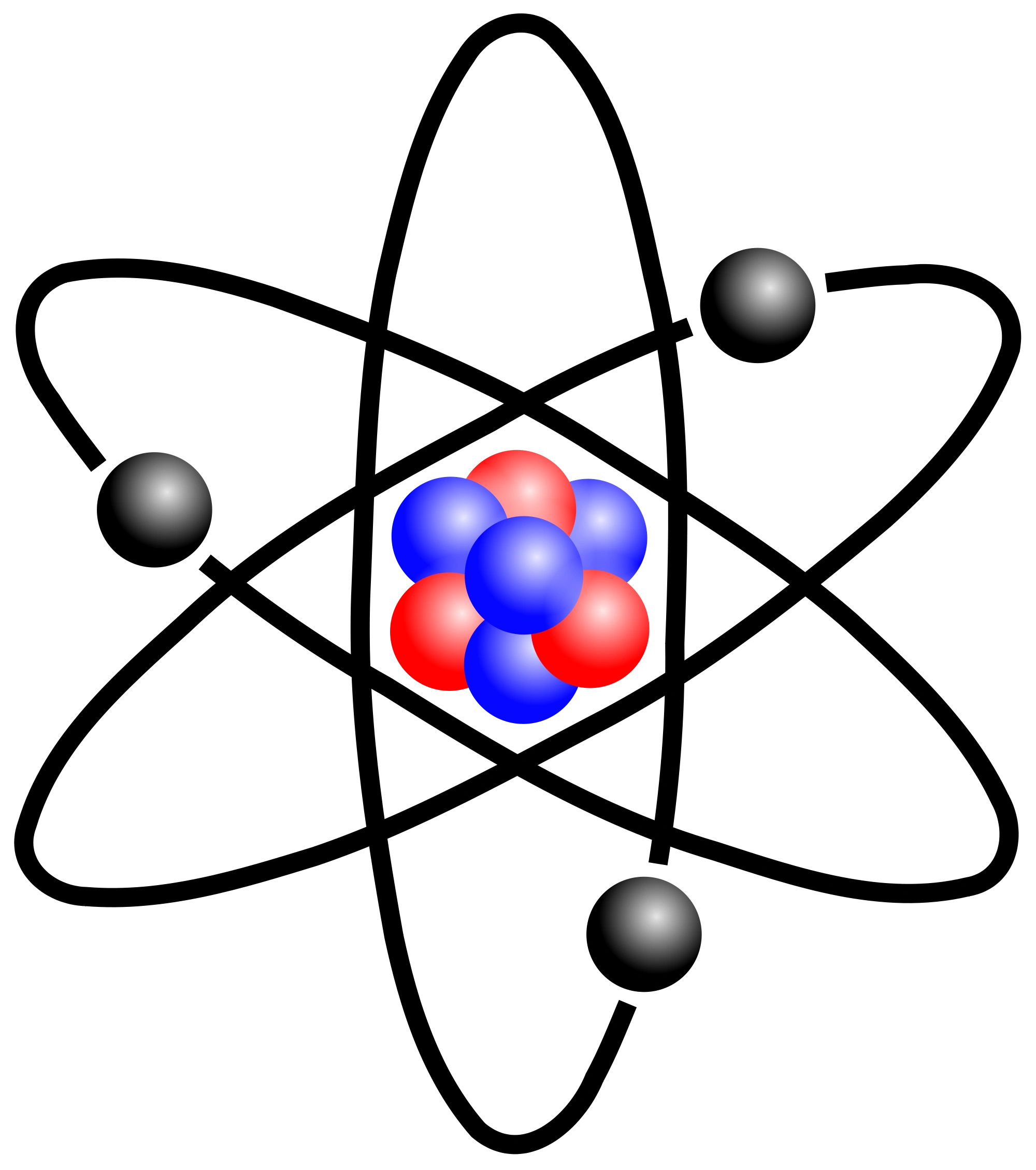
Junior Year Presentation: Building an Atom
- This was our first project for our junior year chemistry class, we were expected to work with a group to be able to create a model that would resemble the atom of an element of the periodic table. We had to include a model that would show the number of neutrons, protons, and electrons of the element, we also had to show and create the types of orbitals that the element’s atom would have, for example S-Orbitals that only contain 2 electrons, P-Orbitals that can only hold 6 electrons, D-Orbitals that can only hold 10 electrons, and finally the F-Orbitals that can only hold 14 electrons. It was very important to be able make the model as accurate as we could because that is what we were being graded on, due to this we had to keep in mind the many theories people had about the model of an atom and how it looked like. We also were expected to make this model interactive so that students would be able to learn with hands on experience.
- This was important to be able to learn because it gives us a overview of Chemist and the study of atoms, elements, and molecules. Not only does it expose us to the idea around an atom but it is important because atoms make up pretty much everything in our planet, there are atoms found everywhere and knowing this allows us know more about our planet and even ourselves because we are also made up of atoms. Groups were required to collaborate with each other and teach each other more about atoms, this would be more of an efficient way to learn because it is hands on, and this allows students to be able to interact with what they are learning and being able to become engaged with the topic and want to learn more. At the end of the project after we had created our models we were able to justify the correct parts of our model along with the parts that seem to be inaccurate because it is impossible to make a perfect model of an atom because we do not know the exact size of the atom or exact position of electrons in an atom.
Comments
Post a Comment